Aug. 12, 2020 | , DOD News
Operation Warp Speed is a public-private partnership whose goal is to deliver 300 hundred million doses of safe and effective COVID-19 vaccines by the end of the year.
The Department of Health and Human Services and the Defense Department are partnered to work with private industry and other federal departments and agencies on this unprecedented effort.
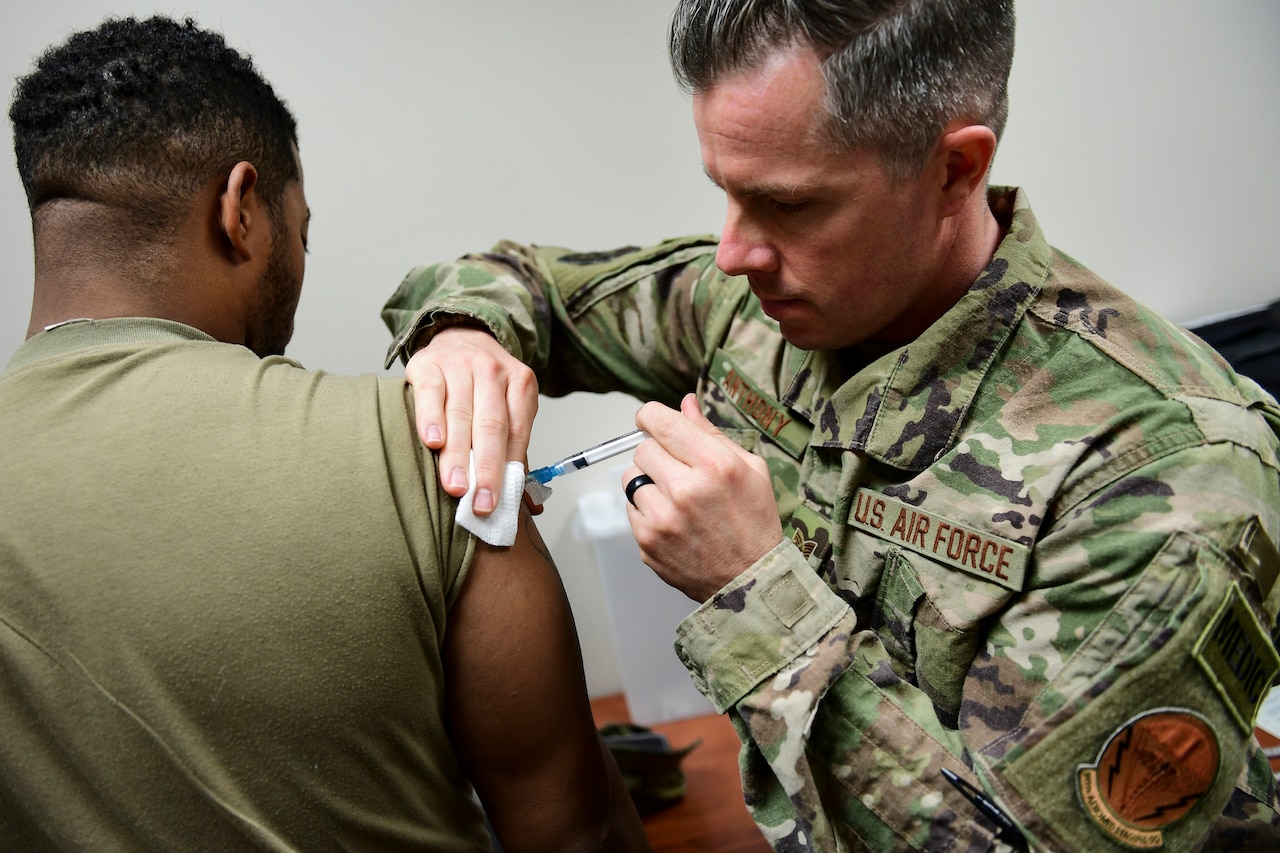
The Operation Warp Speed effort also includes:
- The Centers for Disease Control and Prevention;
- The Food and Drug Administration;
- The National Institutes of Health;
- Biomedical Advanced Research and Development Authority;
- The departments of Agriculture, Energy and Veterans Affairs;
- Industry-leading pharmaceutical research and development, manufacturing and distribution companies; and
- Universities and research institutions.
OWS is well on its way to delivering multiple vaccines to the American people, according to officials involved. Government agencies and private drug firms began working on medical countermeasures when the coronavirus first became known late last year. As a result, multiple vaccine and therapeutic candidates are conducting the later phases of clinical trials needed to demonstrate their safety and efficacy, officials said.
Scaling up that effort once a safe and effective vaccine is developed is the mission of Operation Warp Speed.
OWS aims to deliver 300 million doses of a safe, effective vaccine for COVID-19 by January. Health and Human Services Secretary Alex M. Azar II and Defense Secretary Dr. Mark T. Esper are overseeing the effort. Former pharmaceutical executive Dr. Moncef Slaoui is the chief science advisor for the project, and Army Gen. Gustave F. Perna is the chief operating officer.
Slaoui, an immunologist, has successfully led the development of 14 vaccines.
"I think it will be a very effective vaccine. That's my prediction," Slaoui said. "My personal opinion, based on my experience and the biology of this virus, I think this vaccine is going to be highly efficacious."
Operation Warp Speed will organize multiple, parallel lines of effort, Perna said during his Senate confirmation hearing in June. "Vaccine, therapeutics and diagnostic developments or evaluations are taking place concurrently," he said. "HHS and DOD have already started to increase manufacturing and distribution capacity and capability. Upon approval from [the Food and Drug Administration], Warp Speed will immediately energize manufacturing and distribution networks, in conjunction with industry partners, to speed delivery of those new products to the nation. This is the considered risk we must evaluate and be prepared to take, expanding manufacturing prior to FDA approval."
DOD has led other successful projects, including:
- World War II’s Manhattan Project, which developed the atomic bomb. Army Maj. Gen. Leslie Groves and physicist J. Robert Oppenheimer led the public-private partnership. Scientists conducted research, and the military awarded contracts to build a vast industrial infrastructure.
- A 2007 project to build mine-resistant, ambush-protected vehicles to better protect troops from improvised explosive devices. The vehicles were deployed to the battlefields of Iraq and Afghanistan later that year. More than 12,000 were produced before the program ended in 2012.
Perna said he is committed to streamlining processes that will allow manufacturing and distribution to deliver medical countermeasures at an unprecedented rate. "The collective talent and experience within both the departments of Health and Human Services and Defense, industry and academia must be enabled," he said. "Both industry and our government's supporting agencies, resourcing and execution processes must also move at Warp Speed for success; I will have to ensure that our collective bureaucracies do not distract us from winning."
OWS will ensure 100% of all medical protocols are executed to standard, officials said, adding that science will drive development of candidate countermeasures and the FDA will be the final approval authority.
While speed is crucial, the effectiveness and safety of the vaccines and treatments are matters of paramount importance, Perna has said repeatedly.

Protocols for the demonstration of safety and efficacy are being harmonized, which will allow the trials to proceed more quickly, and the protocols for the trials will be overseen by the federal government, as opposed to traditional public-private partnerships, according to the OWS factsheet from the Department of Health and Human Services.
This will not eliminate steps from the process, but will allow steps to proceed simultaneously, the factsheet explained. Companies will start manufacturing vaccine at an industrial scale well before the demonstration of vaccine efficacy and safety as happens normally. This increases the financial risk, but not the product risk, the factsheet said.
Some vaccines, such as those in development by Moderna and Pfizer, have entered Phase 3 of the testing procedure, Slaoui said. Phase 3 clinical trials began July 27, with 30,000 volunteers slated to enroll across the United States.
These trials, and those of other OWS vaccine candidates, will consist of 30,000 patients in order to allow for rapid collection and earlier analysis of safety and efficacy data by the FDA, he said.
Other vaccines have been identified and are at various stages of testing. The federal government’s investment in the necessary manufacturing capacity gives drug makers confidence that they can invest aggressively in development and will provide for faster distribution of a vaccine if one or more receive FDA authorization or approval, HHS officials said.
OWS is focused on the development, manufacturing and distribution of an FDA-approved, safe and effective vaccine. HHS officials said the prioritization of any COVID-19 vaccines and therapeutics will be determined by CDC recommended allocation methodology used as part of pandemic flu planning and the COVID-19 response.
As the world’s largest organization conducting acquisition and movement of personnel and material, DOD is uniquely capable of meeting the logistical needs of Operation Warp Speed, Perna said, and its involvement in supply, production, and development will enable faster distribution than would have otherwise been possible.
To provide maximum flexibility in support of the ultimate HHS distribution plan, DOD is working with the CDC to have a portfolio of distribution options by August.
"We will offer multiple distribution plans since the outcome depends on, one, the safety and efficacy performance of the vaccines, two, the initial volume and delivery schedule of successful vaccines, and three, the most contemporary understanding of which Americans are most vulnerable as determined by science," Perna said.







No comments:
Post a Comment